Determine an Empirical Formula is a fundamental concept in chemistry, allowing us to uncover the simplest whole-number ratio of atoms in a compound. Understanding the relationship between empirical and molecular formulas is crucial for comprehending the composition of various substances. This guide explores the process from identifying elements to calculating formulas, highlighting crucial techniques and real-world applications.
This in-depth exploration will cover everything from the basic definitions and significance of empirical formulas to practical steps for calculating them from various types of experimental data. We’ll delve into the importance of accurate elemental analysis and the common pitfalls to avoid, ultimately equipping you with a robust understanding of this essential chemical concept.
Introduction to Empirical Formulas
Empirical formulas provide a simplified representation of the elemental composition of a chemical compound. They indicate the simplest whole-number ratio of atoms present in the compound. This is crucial for understanding the fundamental building blocks of a substance and for comparing different compounds with similar compositions. Knowing the empirical formula is essential for determining the molecular formula, which represents the actual number of atoms of each element in a molecule.The relationship between empirical and molecular formulas is directly linked to the relative proportions of elements in a compound.
The empirical formula merely describes the smallest possible ratio, while the molecular formula reveals the precise composition of the molecule. For instance, both glucose (C 6H 12O 6) and formaldehyde (CH 2O) have the same empirical formula (CH 2O). However, their molecular structures and properties differ significantly due to the difference in the number of atoms per molecule.
Figuring out an empirical formula can be tricky, but it’s a fundamental chemistry concept. Thinking about how amazing it is that someone like Beyonce, who has shown tremendous generosity and compassion, donated $82,234 to the Flint water crisis beyonce donates 82234 to flint water crisis , it makes you appreciate the importance of understanding chemical ratios. Ultimately, understanding the empirical formula helps us appreciate the intricate building blocks of everything around us, from the smallest molecules to the grandest of natural disasters.
Significance of Empirical Formulas in Chemical Analysis
Empirical formulas are instrumental in various chemical analysis processes. Determining the empirical formula from experimental data allows chemists to deduce the composition of a compound, aiding in its identification and characterization. This information is fundamental for understanding the properties, reactivity, and potential applications of the substance. For example, analyzing the combustion products of a newly synthesized compound can reveal the empirical formula, providing crucial insight into its composition.
Comparison of Empirical and Molecular Formulas
Understanding the difference between empirical and molecular formulas is essential for interpreting chemical data accurately. This table illustrates the key distinctions between these two types of formulas:
| Feature | Empirical Formula | Molecular Formula |
|---|---|---|
| Definition | Represents the simplest whole-number ratio of atoms in a compound. | Represents the actual number of atoms of each element in a molecule. |
| Information Provided | Ratio of elements. | Precise number of atoms of each element. |
| Examples | H2O (water), CH2O (formaldehyde/glucose) | C6H12O6 (glucose), C2H4O2 (acetic acid) |
| Determination | Often derived from experimental data, like combustion analysis. | Calculated from the empirical formula and the molecular weight. |
Empirical formulas provide a foundation for understanding chemical composition, while molecular formulas offer a more complete picture of molecular structure.
Identifying the Components of a Compound
Unraveling the secrets of a chemical compound starts with identifying its constituent elements. This crucial step underpins the entire process of determining an empirical formula, as it dictates the specific elements and their relative proportions. Accurate identification is paramount, as even a minor error can lead to a flawed formula and subsequently, an inaccurate representation of the compound’s composition.Determining the elements present in a compound is achieved through a combination of qualitative and quantitative analysis techniques.
Qualitative analysis identifies the
- what*—which elements are present. Quantitative analysis determines the
- how much*—the relative amounts of each element. These analyses, when performed meticulously, allow for the calculation of the empirical formula.
Methods for Elemental Analysis, Determine an Empirical Formula
Precise elemental analysis is essential for obtaining accurate empirical formulas. Various methods are available, each with its strengths and limitations. The choice of method depends on the nature of the substance being analyzed and the desired level of precision.
- Chemical Tests: A suite of chemical tests can be employed to identify specific elements. For instance, the presence of halogens can be detected by using silver nitrate solutions, while the presence of certain metals can be identified through flame tests, which cause different colors depending on the metal.
- Spectroscopic Techniques: Spectroscopy provides a powerful means of identifying elements based on their unique interactions with electromagnetic radiation. Techniques like atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES) measure the absorption or emission of light by atoms, allowing for precise identification of elements. Mass spectrometry (MS) is another important technique that measures the mass-to-charge ratio of ions, enabling the determination of elemental composition.
Figuring out an empirical formula can be tricky, but it’s a fundamental chemistry concept. Think about how meticulously a real-life spy like the women profiled at black widow real life spy needs to calculate the exact ratios of elements in their plans, or how much poison is needed. Ultimately, determining an empirical formula is about finding the simplest whole-number ratio of elements in a compound, just like a spy’s most effective strategy involves precision and calculation.
These methods often provide quantitative data alongside qualitative identification.
- X-ray Diffraction: X-ray diffraction (XRD) is a valuable tool for analyzing crystalline solids. By measuring the diffraction patterns of X-rays scattered by the crystal lattice, the presence and arrangement of atoms within the solid can be determined. This information is instrumental in understanding the structure of compounds and identifying the constituent elements.
Importance of Accurate Elemental Analysis
Accurate elemental analysis is fundamental to obtaining the correct empirical formula. A small error in the percentage composition of an element can lead to a significant deviation in the calculated formula. For instance, in the synthesis of a pharmaceutical compound, an incorrect empirical formula could lead to a medication with a different therapeutic effect or even potentially harmful side effects.
The determination of the exact elemental composition of a compound is critical in various scientific fields, including materials science, environmental science, and pharmaceutical chemistry. It is essential for both understanding the properties of a substance and for accurately representing its composition in chemical equations and formulas.
A Comparison of Elemental Analysis Methods
| Method | Description | Strengths | Limitations |
|---|---|---|---|
| Chemical Tests | Based on specific reactions with reagents. | Relatively inexpensive, simple to perform. | Less precise, may not be applicable to all elements or compounds. |
| Spectroscopic Techniques (e.g., AAS, AES, MS) | Measure interactions with electromagnetic radiation. | High precision and sensitivity, applicable to a wide range of samples. | Can be complex and expensive, may require specialized equipment. |
| X-ray Diffraction (XRD) | Analyzes crystalline solids based on diffraction patterns. | Provides structural information alongside elemental identification. | Limited to crystalline materials, may require specialized expertise. |
Calculating Empirical Formulas from Experimental Data
Unveiling the simplest whole-number ratios of elements in a compound is a crucial step in chemical analysis. Empirical formulas provide this essential information, derived from experimental data. Understanding how to calculate them from various experimental setups, like percent composition, mass data, and combustion analysis, empowers us to determine the fundamental makeup of a compound.
Calculating Empirical Formulas from Percent Composition Data
Percent composition data represents the percentage by mass of each element in a compound. To derive the empirical formula, we assume a 100-gram sample, enabling us to directly translate the percentages into grams.
- Convert percentages to grams: If a compound is 40% carbon and 60% oxygen by mass, a 100-gram sample will contain 40 grams of carbon and 60 grams of oxygen.
- Convert grams to moles: Utilize the molar mass of each element to determine the number of moles of each element present. For instance, the molar mass of carbon (C) is approximately 12.01 g/mol, and the molar mass of oxygen (O) is approximately 16.00 g/mol.
- Determine the mole ratio: Divide the number of moles of each element by the smallest number of moles calculated in the previous step. This ratio represents the simplest whole-number ratio of the elements in the compound.
- Express as subscripts in the formula: The resulting mole ratio becomes the subscripts in the empirical formula.
Calculating Empirical Formulas from Mass Data
Given the mass of each element in a sample, the calculation procedure is similar to the percent composition method.
- Convert masses to moles: Use the molar mass of each element to determine the number of moles of each element present.
- Determine the mole ratio: Divide the number of moles of each element by the smallest number of moles calculated in the previous step.
- Express as subscripts in the formula: The resulting mole ratio becomes the subscripts in the empirical formula.
Determining Empirical Formulas from Combustion Analysis
Combustion analysis is a technique used to determine the elemental composition of a compound by burning it in a controlled environment and measuring the mass of the products.
- Record the mass of carbon dioxide (CO2) and water (H 2O) produced: This data provides information about the carbon and hydrogen content of the original compound.
- Calculate the moles of carbon (C) and hydrogen (H): The mass of CO 2 produced is directly related to the amount of carbon in the original sample. Similarly, the mass of H 2O produced is related to the hydrogen content. Using the molar masses of CO 2 and H 2O, calculate the moles of carbon and hydrogen present.
- Determine the mass of oxygen (O): The mass of oxygen can be determined by subtracting the mass of carbon and hydrogen from the total mass of the original sample.
- Convert the masses of C, H, and O to moles: Utilize the molar masses of C, H, and O to find the number of moles of each element present.
- Determine the mole ratio: Divide the number of moles of each element by the smallest number of moles.
- Express as subscripts in the formula: The resulting mole ratio becomes the subscripts in the empirical formula.
Examples
Example 1: A compound is 75% carbon and 25% hydrogen. Find the empirical formula.
Solution: Assume a 100g sample. 75g C, 25g H. Moles C = 75g / 12.01 g/mol = 6.24 mol; Moles H = 25g / 1.01 g/mol = 24.75 mol. Ratio C:H = 6.24/6.24 : 24.75/6.24 ≈ 1:
4. Empirical formula: CH 4
Example 2: Combustion analysis of a 0.500 g sample yields 1.10 g of CO 2 and 0.450 g of H 2O. Find the empirical formula.
Solution: Moles CO 2 = 1.10 g / 44.01 g/mol = 0.0250 mol; Moles C = 0.0250 mol. Moles H 2O = 0.450 g / 18.02 g/mol = 0.0250 mol; Moles H = 0.0500 mol. Ratio C:H = 0.0250/0.0250 : 0.0500/0.0250 ≈ 1:
2. Empirical formula: CH 2.
Dealing with Fractional Subscripts in Empirical Formulas
Determining the empirical formula of a compound from experimental data often leads to fractional subscripts for some elements. These fractions arise because the initial ratios of the elements might not immediately translate into whole numbers, representing the simplest atomic ratio. This situation requires a specific approach to ensure the formula accurately reflects the fundamental composition of the compound.
Fractional Subscript Handling Procedures
To convert fractional subscripts into whole numbers, a crucial step in empirical formula calculations, we employ a common mathematical technique. This method ensures the resulting empirical formula represents the smallest whole-number ratio of atoms within the compound. Multiplying all subscripts by a suitable factor allows us to obtain integer values.
Examples of Compounds with Fractional Subscripts
Many compounds, especially those involving non-integer ratios of elements during analysis, yield fractional subscripts in the initial calculation stage. Consider copper(I) oxide, for example, where the initial experimental data might suggest a ratio of copper to oxygen atoms that isn’t an exact whole number. Similarly, compounds like iron(II) oxide or iron(III) oxide may have initially fractional ratios during analysis, requiring the conversion process to obtain their correct empirical formulas.
Methods for Converting Fractional Subscripts to Whole Numbers
The key to converting fractional subscripts lies in identifying the least common multiple (LCM) of the denominators. This LCM value acts as the multiplier to convert all the fractional subscripts to whole numbers. The LCM ensures that all the ratios in the formula are in their simplest whole-number form, reflecting the actual atomic proportions in the compound.
Figuring out an empirical formula can be tricky, but it’s a fundamental chemistry concept. Thinking about how atoms combine to form molecules is key. Listening to bands like thalia zedek band walk away helps me appreciate how different elements can come together to create something new and unique, just like chemical reactions! Ultimately, determining an empirical formula is about uncovering the simplest whole-number ratio of atoms in a compound.
Illustrative Table
| Compound Composition (Initial Ratio) | Fractional Subscripts | Least Common Multiple (LCM) | Multiplied Subscripts | Empirical Formula |
|---|---|---|---|---|
| Element A: 0.5, Element B: 1.0 | A0.5B1.0 | 2 | A1B2 | AB2 |
| Element C: 0.75, Element D: 1.0 | C0.75D1.0 | 4 | C3D4 | C3D4 |
| Element E: 1.5, Element F: 1.0 | E1.5F1.0 | 2 | E3F2 | E3F2 |
| Element G: 2.5, Element H: 1.0 | G2.5H1.0 | 2 | G5H2 | G5H2 |
The table demonstrates the procedure: We find the LCM of the denominators of the fractional subscripts. This LCM becomes the multiplier for all subscripts, resulting in a formula with only whole numbers. This method ensures the empirical formula accurately represents the simplest whole-number ratio of atoms in the compound.
Understanding Limitations and Assumptions
Empirical formulas provide a valuable snapshot of a compound’s elemental composition, but they don’t reveal the complete molecular structure. This simplicity comes with limitations. Understanding these limitations and the assumptions underlying empirical formula calculations is crucial for interpreting the results correctly. Knowing the limitations allows for a more nuanced understanding of the information an empirical formula provides and its limitations.The empirical formula only represents the simplest whole-number ratio of atoms in a compound.
It doesn’t tell us about the arrangement of these atoms, the bond types, or the overall shape of the molecule. For instance, both benzene (C 6H 6) and acetylene (C 2H 2) have the same empirical formula (CH), yet their structures and properties differ significantly. Thus, an empirical formula provides a starting point, but a complete understanding of a compound’s nature requires further experimental techniques.
Limitations of Empirical Formulas
Empirical formulas, by their nature, cannot fully describe the molecular structure of a substance. They only give the relative proportions of the elements present, not the specific arrangement or bonding within the molecule. This limitation is critical because different molecules can have the same empirical formula but very different properties. The same formula, for example, can describe various isomers with differing properties.
Assumptions Made in Calculations
Accurate empirical formula calculations rely on several key assumptions. One critical assumption is that the experimental data accurately reflects the true composition of the compound. Errors in sample preparation, measurement, or analysis can introduce inaccuracies. Another crucial assumption is the complete reaction of all reactants, without any side products or unreacted material. Finally, precise and accurate measurements of the masses of the elements are essential.
Small errors in mass measurement can lead to significant deviations in the calculated empirical formula.
Possible Sources of Error
Several factors can contribute to errors in determining empirical formulas. These errors can arise from the experimental process itself or from the assumptions made during calculation. Incomplete reactions, impure samples, and inaccurate mass measurements are all potential sources of error. For example, if a sample contains impurities, the calculated proportions of elements will be incorrect. Additionally, if the reaction is not complete, the calculated empirical formula might not reflect the true composition of the compound.
The precision of the analytical balance used for weighing will also influence the accuracy of the final result.
Summary Table of Potential Limitations
| Potential Limitation | Impact on Results |
|---|---|
| Incomplete reaction | Incorrect elemental ratios in the calculated formula. |
| Impure sample | Inaccurate elemental ratios due to the presence of other elements or compounds. |
| Inaccurate mass measurements | Significant deviation from the true empirical formula. |
| Experimental error in analysis | Misrepresentation of the elemental composition. |
| Incorrect assumptions about the stoichiometry of the reaction | Misleading or inaccurate results, especially if the reaction is not complete or side reactions occur. |
Illustrative Examples and Applications
Empirical formulas are fundamental tools in chemistry, providing crucial insights into the composition of compounds. Understanding how to calculate them from experimental data is essential for various scientific endeavors, from analyzing unknown substances to understanding complex chemical reactions. This section delves into practical examples and the widespread applications of empirical formulas in different scientific fields.Calculating empirical formulas involves several steps, starting with experimental data like the masses of elements present in a compound.
These masses are converted into moles, and then the mole ratios are used to determine the simplest whole-number ratio of the elements. This process provides valuable information about the makeup of a substance.
Calculating Empirical Formulas from Experimental Data
Determining the empirical formula from experimental data is a common practice in analytical chemistry. The procedure involves carefully measuring the mass of each element in a compound. Let’s consider a few examples:
- Example 1: A compound is found to contain 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen by mass. To find the empirical formula, first assume a 100 g sample. This gives 40.0 g carbon, 6.7 g hydrogen, and 53.3 g oxygen. Next, convert these masses to moles using the molar masses of each element (C = 12.01 g/mol, H = 1.01 g/mol, O = 16.00 g/mol).
- Moles of C = 40.0 g / 12.01 g/mol ≈ 3.33 moles
- Moles of H = 6.7 g / 1.01 g/mol ≈ 6.63 moles
- Moles of O = 53.3 g / 16.00 g/mol ≈ 3.33 moles
Dividing each mole value by the smallest value (3.33) yields the mole ratio of 1:2:1, leading to the empirical formula CH 2O.
- Example 2: A chemist analyzes a sample of a hydrate and finds 18.3 g of anhydrous salt and 2.7 g of water. The molar mass of the anhydrous salt is 142 g/mol and the molar mass of water is 18.0 g/mol.
- Moles of anhydrous salt = 18.3 g / 142 g/mol ≈ 0.129 moles
- Moles of water = 2.7 g / 18.0 g/mol ≈ 0.15 moles
Dividing by the smallest value (0.129), gives the mole ratio of 1:1.
17. Multiplying both by 3 to get whole numbers results in a ratio of 3
4, hence the empirical formula is X 3H 4O.
Applications of Empirical Formulas in Various Fields
Empirical formulas provide a foundation for understanding the composition of countless substances in diverse fields. Their importance extends far beyond basic chemistry.
- Material Science: Identifying the empirical formula of a new polymer or composite material is essential for understanding its properties and potential applications. This information guides the development of novel materials with desired characteristics, such as strength, flexibility, or conductivity.
- Environmental Science: Determining the empirical formula of pollutants in air or water samples is crucial for understanding their impact on ecosystems and human health. This aids in developing strategies for pollution control and remediation.
- Medicine: Empirical formulas are essential in pharmaceutical research, where they are used to determine the precise composition of drugs. This knowledge is vital for ensuring the drug’s effectiveness and safety.
Real-World Scenarios
Empirical formula determination is critical in various real-world scenarios.
- Forensic Science: Analyzing the empirical formula of substances found at a crime scene can help in identifying unknown materials, linking suspects to a crime, or understanding the process of a chemical reaction.
- Archaeology: Determining the empirical formula of ancient artifacts can provide insights into the materials used and the techniques employed in their creation.
Table of Applications
| Field | Application |
|---|---|
| Material Science | Developing new materials with desired properties |
| Environmental Science | Understanding and controlling pollution |
| Medicine | Formulating safe and effective drugs |
| Forensic Science | Identifying unknown substances at crime scenes |
| Archaeology | Understanding ancient materials and techniques |
Illustrative Examples in Visual Form
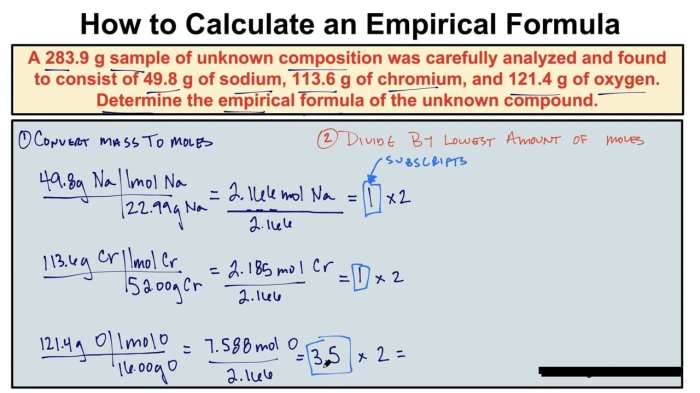
Visual representations significantly aid in understanding the process of determining empirical formulas. These visual aids clarify the steps involved in converting experimental data to the simplest whole-number ratio of elements in a compound. The following examples showcase different approaches to this calculation, making the concept more accessible and tangible.
Visual Representation of Empirical Formula Calculation
Visualizing the steps involved in calculating an empirical formula makes the process much clearer. Consider the following example: A compound is found to contain 52.17% carbon and 47.83% hydrogen. We can use a table to organize the information and guide the calculations.
| Element | Mass % | Moles | Mole Ratio | Simplified Ratio |
|---|---|---|---|---|
| Carbon (C) | 52.17 | 52.17 g / 12.01 g/mol = 4.34 mol | 4.34 mol / 4.34 mol = 1 | 1 |
| Hydrogen (H) | 47.83 | 47.83 g / 1.01 g/mol = 47.1 mol | 47.1 mol / 4.34 mol = 10.9 | 11 |
A visual representation of this process might be a flowchart, starting with the mass percentages. The next step would involve converting the percentages to moles, using the molar masses of each element. The table demonstrates the mole ratio calculated from the moles of each element. Finally, the simplified whole-number ratio is derived.
Example: Combustion Analysis and Empirical Formula
Imagine analyzing a hydrocarbon sample using combustion analysis. The experiment produces 2.20 grams of CO 2 and 0.900 grams of H 2O. Visualizing this process involves understanding the relationship between the products (CO 2 and H 2O) and the initial hydrocarbon. This example demonstrates how to determine the empirical formula from the mass of the products.
To determine the empirical formula, we need to determine the moles of carbon and hydrogen present in the compound.
- From the mass of CO 2, we calculate the moles of carbon. The visual representation of this step might involve a diagram showing the conversion of mass of CO 2 to moles of carbon.
- Similarly, we calculate the moles of hydrogen from the mass of H 2O. The visual representation might include a diagram showing the conversion of mass of H 2O to moles of hydrogen.
- The next step is to establish the mole ratio between carbon and hydrogen. A visual could show a comparison of the moles of carbon and hydrogen, perhaps with a graph or a double-axis bar chart, highlighting the ratio.
- The final step involves simplifying the mole ratio to the smallest whole numbers, providing the empirical formula. This step can be represented by a simple table summarizing the calculation and the final empirical formula.
Chemical Reaction and Visual Representation
Consider the reaction of methane (CH 4) with oxygen (O 2) to produce carbon dioxide (CO 2) and water (H 2O). The visual representation of this reaction could be a molecular model illustrating the rearrangement of atoms during the reaction.
The empirical formula of the reactants and products can be determined by counting the atoms of each element.
A diagram showing the molecules of methane, oxygen, carbon dioxide, and water, with the atoms labeled, would effectively illustrate the reaction. The number of atoms in each molecule should be clearly indicated, highlighting the conservation of mass in the chemical reaction. Such a visual aid could be a series of labeled diagrams, before and after the reaction, demonstrating the transformation of reactants into products.
End of Discussion: Determine An Empirical Formula
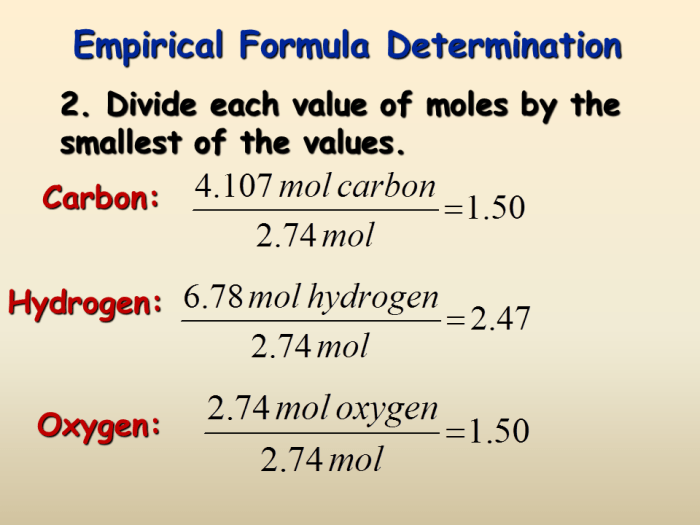
In conclusion, determining an empirical formula is a cornerstone of chemical analysis, enabling us to unveil the elemental makeup of compounds. From percent composition data to combustion analysis, the methods described provide a robust framework for calculating these formulas. While there are limitations and potential sources of error, this guide equips you with the knowledge to tackle these calculations confidently and apply your understanding to various chemical scenarios.

Leave a Reply